Background
Allogeneic hematopoietic stem cell transplants (alloSCT) offer the only curative treatment for many hematological cancers but carry the risk of significant toxicity including graft versus host disease (GvHD). Additionally, while the use of a myeloablative conditioning (MAC) regimen optimizes disease control, it can increase the complications associated with transplant and non-relapse mortality. Therefore, older patients are often deemed unfit for MAC and relegated to treatment with reduced intensity conditioning (RIC) regimens, which result in a higher incidence of relapse.
Orca-T is a high-precision cell therapy biologic that includes stem and immune cells, derived from allogeneic donors, that leverages highly purified, polyclonal donor regulatory T cells to control alloreactive immune responses. The specific subsets selected in the Orca-T manufacturing process retain cells with therapeutic benefit while removing those that pose potential risks, allowing for intensification of concomitant chemotherapy and resulting in overall positive outcomes to date in the phase 1b trial (NCT04013685). To assess the safety and efficacy in an older patient population, we compared outcomes in those at least 55 years old to younger patients treated with Orca-T, receiving chemotherapy-based MAC with tacrolimus as single-agent GvHD prophylaxis.
Methods
As of 6/02/23, 38 patients ≥18 to <55 (younger) and 25 patients ≥55 years of age (older) received Orca-T as part of a multicenter Phase 1b single-arm trial and had the following diagnosis: acute myeloid, lymphoid or mixed phenotype leukemia in CR/CRi, myelodysplastic syndrome or chronic myeloid leukemia that was in chronic phase. Patients with active disease at the time of transplantation and patients with myelofibrosis were not included in this analysis. Patients received a myeloablative regimen consisting of intravenous busulfan, fludarabine, and thiotepa (BFT) prior to Orca-T, followed by single-agent GvHD prophylaxis with tacrolimus, and had an 8/8 related or unrelated matched donor. Donors were matched via DNA-based high-resolution typing of HLA-A, -B, -C, and -DRB1.
Results
Orca-T was successfully manufactured at a centralized GMP facility, distributed and infused at study sites throughout the U.S. Vein-to-vein time (i.e. time between end of donor apheresis to start of recipient's Orca-T infusion) was < 72 hours for all patients, majority < 60 hours.
Median age was 47 in the younger group and 59 in the older group. The younger group was 50% female, and the older group was 36% female. The percentage that were of Hispanic or Latino ethnicity were 11% in the younger group, and 12% in the older group. 8%, 74% and 11% vs 12%, 44% and 32% had ALL, AML and MDS in the younger and older groups, respectively. A greater proportion of patients in the younger group had a baseline HCT-CI score of 0 (53%), while more had a baseline HCT-CI score of 4 (12%) in the older group. The median duration of follow-up for both groups was 12 months (Table).
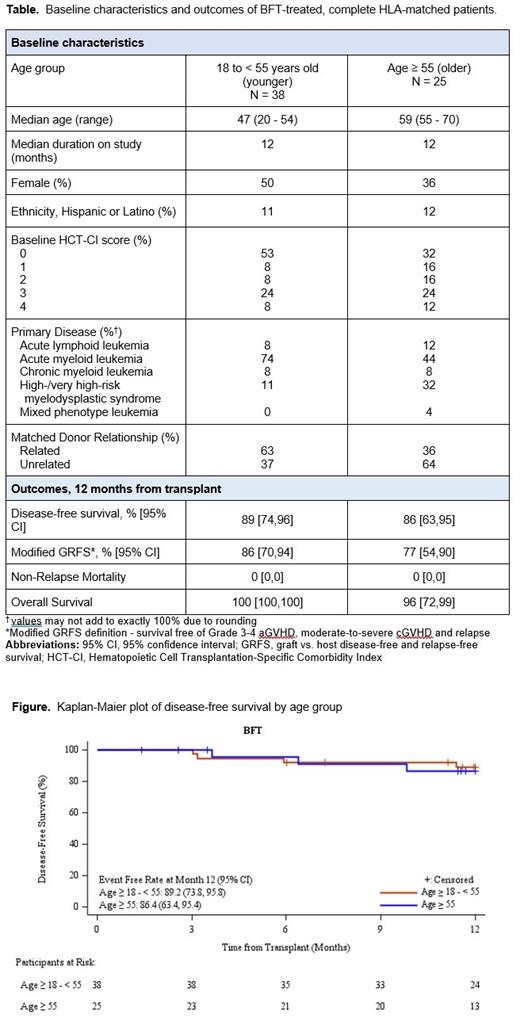
Disease free survival at 1 year was similar in both groups at 89% in the younger patients and 86% in the older patients (Figure). Modified GRFS* rate at 12 months among patients in the younger group was 86% compared to 77% in the older group. Non-relapse mortality at 1 year was 0% in both of these groups who received BFT MAC. Overall survival was high at 100% and 96% at 12 months in the younger and older patients, respectively.
There were two patients > 65 when they received Orca-T; both remain alive without evidence of grade 3-4 acute GvHD, moderate to severe cGvHD, or relapse to date.
Only 2 patients experienced grade 3 mucositis (no grade 4-5) and 1 younger patient (2.6%) and 1 older patient (4.0%) required total parenteral nutrition.
Conclusions
Our analysis suggests that Orca-T cell therapy represents a reduced toxicity alternative to conventional transplant and is well-tolerated by patients 55 and older, with comparable clinical outcomes to those 18 - < 55. In both patient groups, relapse incidence was low, with high GRFS. Remarkably, non-relapse mortality was zero at 1 year with excellent overall survival , suggesting that this regimen could potentially improve outcomes for older patients. An ongoing Phase 3 trial evaluating Orca-T vs. standard of care is currently enrolling throughout the United States.
Disclosures
Oliai:Novartis: Research Funding; Jazz Pharmaceuticals: Research Funding; Arog: Research Funding; Seagen: Research Funding; Orca Bio: Research Funding; Pfizer: Research Funding. Pantin:Omeros, Sanofi: Speakers Bureau; Orca Bio: Research Funding; Omeros: Honoraria. Hoeg:Orca Bio: Research Funding. Muffly:pfizer: Consultancy; kite: Consultancy, Honoraria, Research Funding; autolus: Consultancy; astellas: Consultancy, Research Funding; jasper: Research Funding; bms: Research Funding; adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; orca bio: Research Funding; amgen: Consultancy. Patel:orca bio: Research Funding; Sanofi: Speakers Bureau; CTi BioPharma: Consultancy; Kite Pharma: Speakers Bureau. Gandhi:orca bio: Research Funding. Lowsky:Orca Bio: Research Funding. Salhotra:Jazz Pharma: Research Funding; Rigel Pharma: Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees; Sanofi: Speakers Bureau; OrcaBio: Research Funding; BMS: Research Funding; Kura Oncology: Research Funding; Gilead: Research Funding. Dholaria:Lumanity: Consultancy; Wugen: Research Funding; Gilead: Research Funding; ADC therapeutics: Consultancy, Honoraria; Arivan: Consultancy; Boxer Capital: Consultancy; Pluri Biotech: Consultancy; AstraZeneca: Research Funding; Molecular Templates: Research Funding; Atara: Research Funding; NCI: Research Funding; Allovir: Research Funding; Poseida: Research Funding; Orca Bio: Research Funding; MEI: Research Funding; Adicet: Research Funding; Angiocrine: Research Funding; Takeda: Research Funding; Pfizer: Research Funding; BEAM therapeutics: Consultancy; gamida cel: Consultancy; Janssen: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Ellipsis pharma: Consultancy; Poseida: Research Funding. Waller:Verastem: Consultancy, Research Funding; Allovir: Consultancy; CRISPR: Consultancy; Novartis: Consultancy, Research Funding; CSL Behring: Consultancy, Research Funding; ORCA: Research Funding; BMS: Research Funding; Sanofi: Research Funding; NCI R01: Research Funding; Secura: Research Funding; PartnersTherapeutics: Research Funding; Cambium Medical Technologies: Current equity holder in private company, Other: Founder; Cambium Oncology: Current equity holder in private company, Other: Founder. Srour:Orca Bio: Research Funding. Pavlova:Orca Bio: Current Employment. Fernhoff:Orca Bio: Current Employment. Agodoa:Orca Bio: Current Employment. McClellan:Orca Bio: Current Employment. Abedi:Orca Bio: Research Funding. Negrin:Appia Bio: Membership on an entity's Board of Directors or advisory committees; Regimmune, Inc.: Consultancy; Co-Immune: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Orca Bio: Research Funding; UpToDate: Patents & Royalties; Cellenkos: Consultancy; Biorasi: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Garuda Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Meyer:Orca Bio: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal